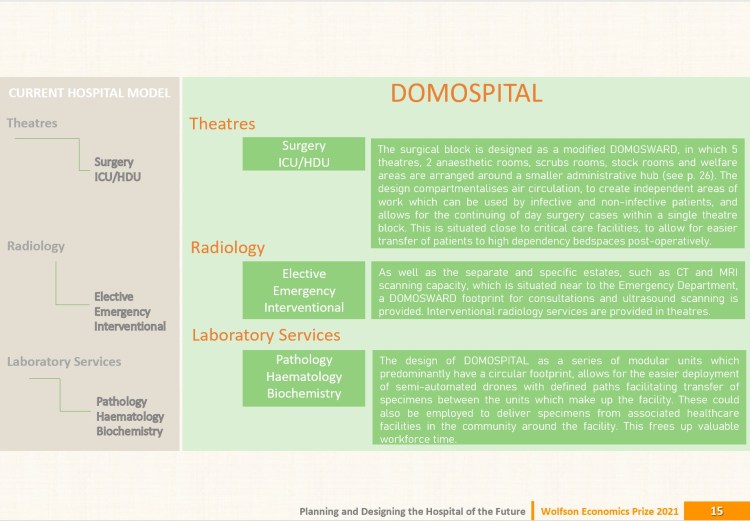
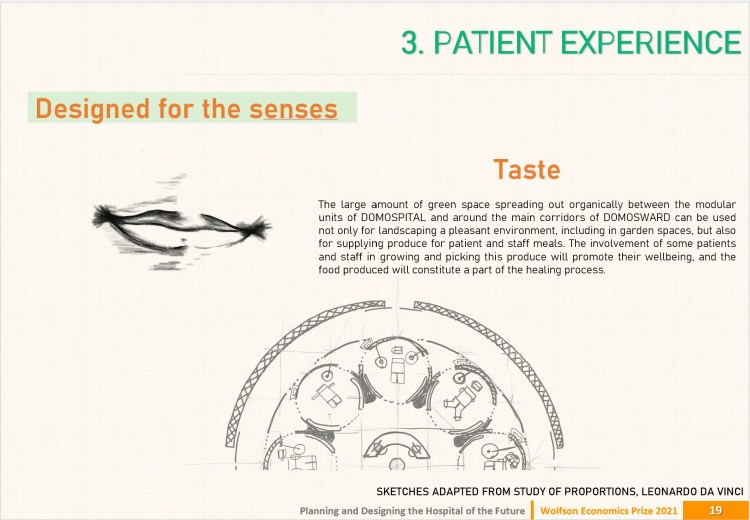
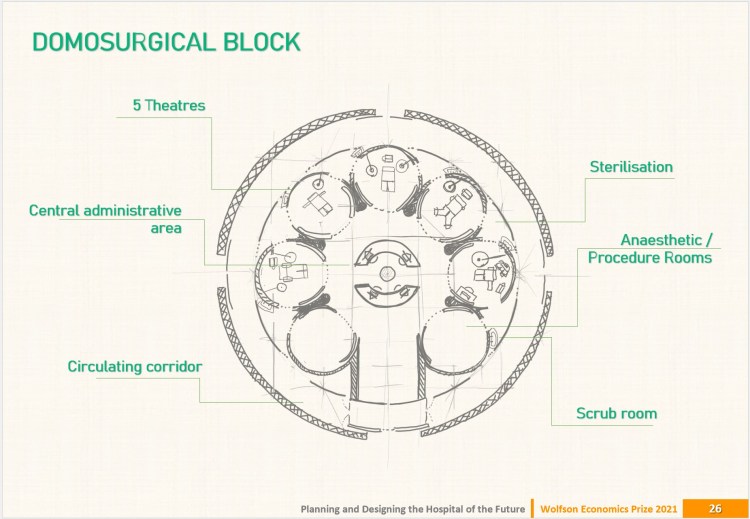
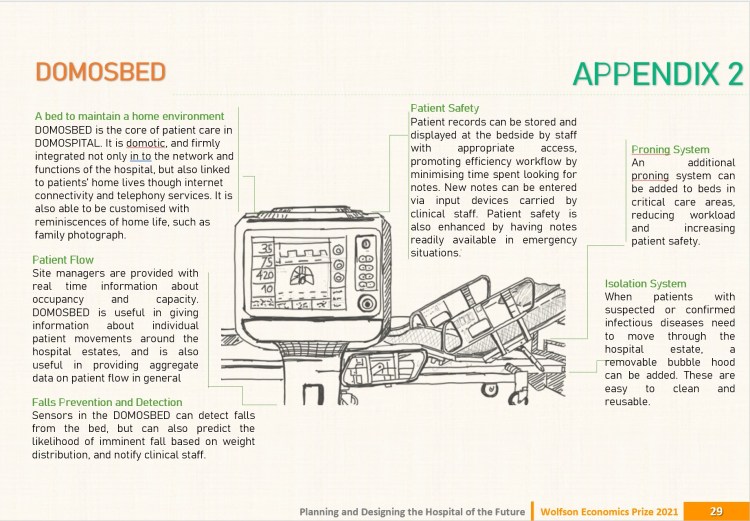
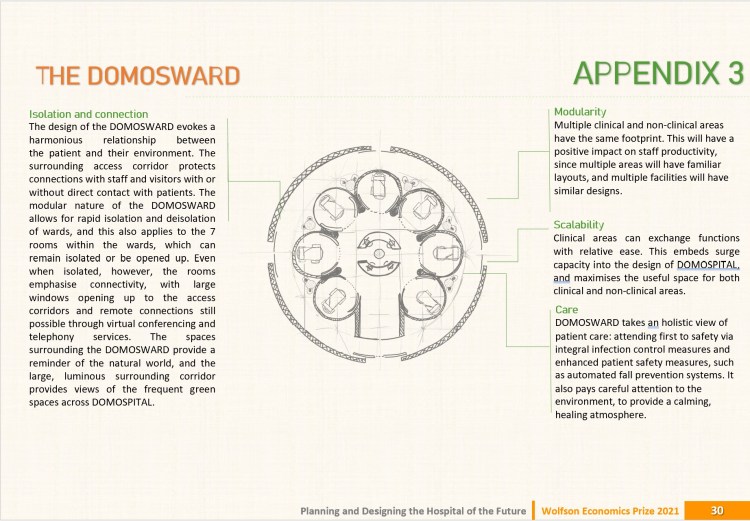
DOMOSPITAL by J. Kelly & J. Martin
Planning and Designing the Hospital of the Future.


GASTRIC ADHERING POLYMER FOR SEMAGLUTIDE
Abstract
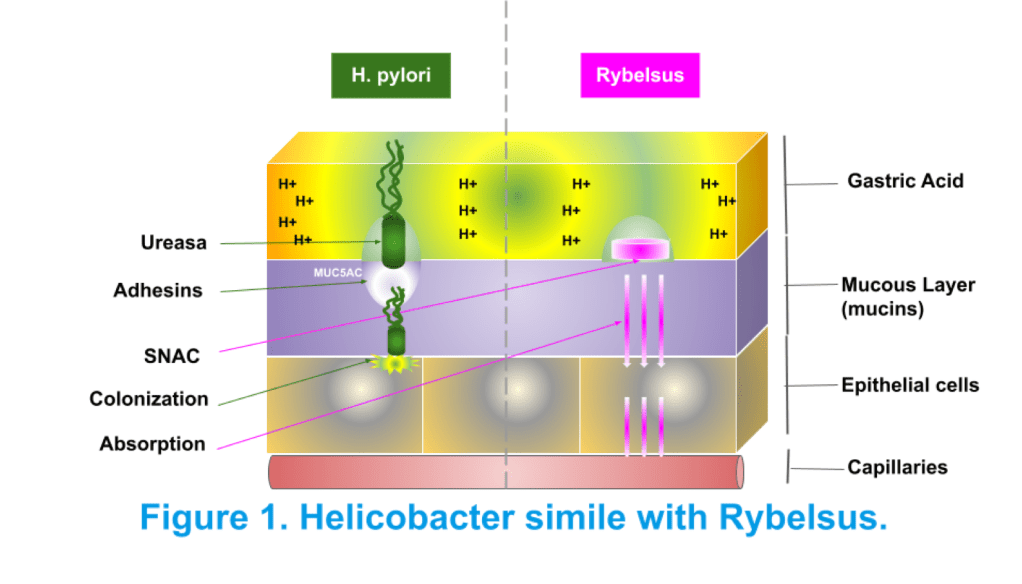
We present GAPS (Gastric Adhering Polymer Solution) as an innovative method to optimize the formulation of the oral peptide drug semaglutide (Rybelsus®). After an extensive literature review and rigorous process of analysis from the initial semaglutide molecule we can conclude that Novo Nordisk’s oral semaglutide is a safe and efficacious molecule hitting the correct target. Moreover, the study carried by Buckey et al., confers robust evidence that transepithelial absorption through the transcellular route is effective. However, we could identify the problems facing the absorption of the molecule with the concomitant administration of food allowing us to provide a solution based on the selective adhesion of the oral semaglutide tablet to the gastric mucus rich in mucins. Conjugating semaglutide – SNAC – GAPS we obtain a functionalized biopolymer in a form of microspheres that targets mucin type MUC5AC and achieve a selective fixation of semaglutide that could not be displaced by food, fluids or other drugs. GAPS provides a selective adhesion to human gastric parietal cells avoiding fluid – food dispersion effect on the semaglutide in the stomach, maintaining the protective effects of SNAC against proteolytic enzymes and increasing the remaining time on the gastric pit to enhance absorption of semaglutide through the ranscellular route. The creation of GAPS was inspired on biomimicry with the firm adhesion mechanism of the Helicobacter pylori bacteria which selectively adheres to the mucin type MUC5AC, very abundant at the gastric pit, where semaglutide is absorbed, in the same place where the bacteria penetrates to the parietal cell to parasite the stomach. GAPS can be formulated in thiolated chitosan nanoparticles intended to be administered any time of day, swallowed through the enteral route, with the potential to perform better than the current oral dosage form of semaglutide and being able to be taken with other oral medications. Our solution is based upon the current formulation in a solid oral dosage with the dimensions of 7.5 mm 13.5 mm. GAPS facilitates oral absorption of semaglutide and is stable at room temperature, with good organoleptic properties. As well as being environmentally friendly and contains ingredients that are generally recognized as safe. GAPS allows the seeker to manufacture the active principle on a large – scale and provides the seeker freedom to practice and is available for potential licensing. There is no third party patent art so commercial application is subject only to our agreement.
Note: more detailed information on this project can be provided upon request.